VALIDAÇÃO, INCERTEZA E
CONTROLE DE QUALIDADE
CONTROLE DE QUALIDADE
ConfLab Validação
O software ConfLab Validação realiza inúmeros cálculos, em diferentes parâmetros, focando no atendimento de guias ou normas para validação de métodos e foi desenvolvido com foco na nova ISO 17025:2017. A ideia é que o usuário somente entre com os valores experimentais e o software, automaticamente, calcula os parâmetros, apresenta os resultados e permite a impressão de um relatório de validação totalmente personalizável, com todos os dados de rastreabilidade exigidos pelos sistemas de gestão da qualidade. É também compatível e atende as exigências das Boas Práticas de Laboratório (BPL). Dentre os parâmetros para a Validação de Métodos destacam-se:
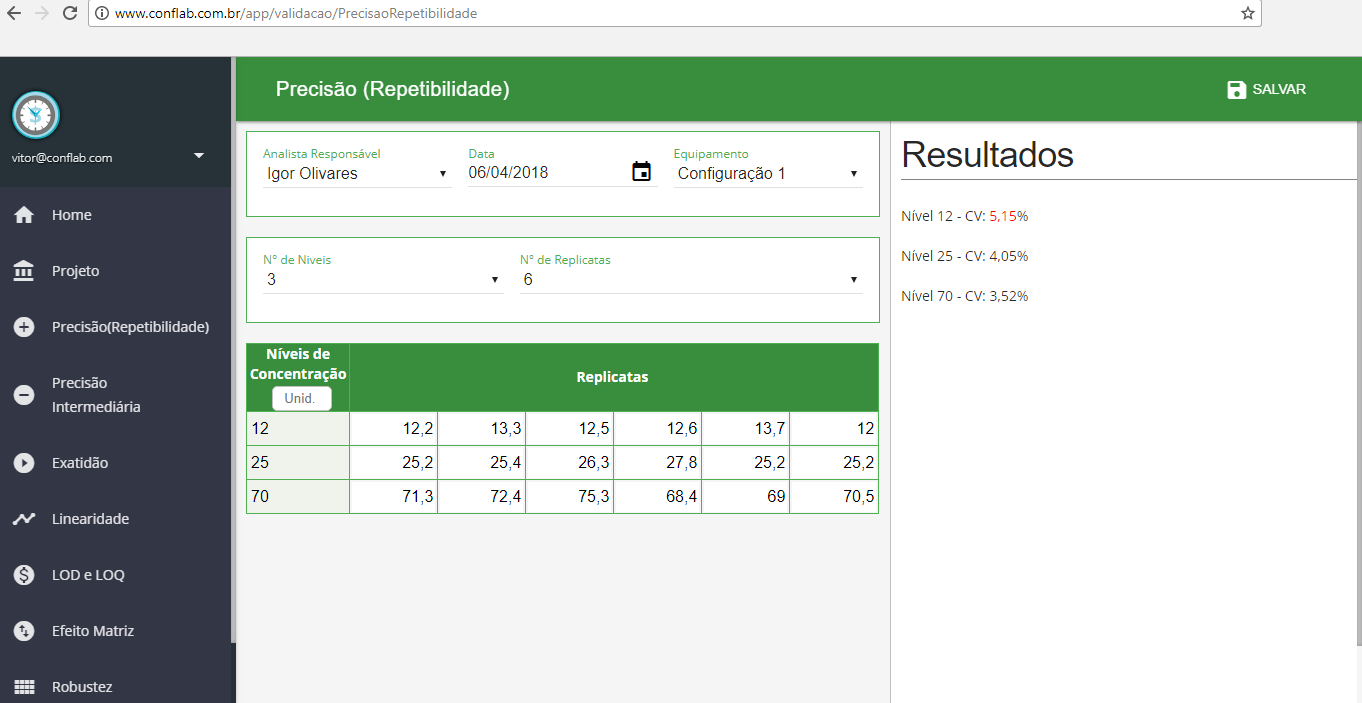
- Precisão (Repetibilidade)
- Precisão Intermediária
-
Exatidão
- Exatidão com Amostras Fortificadas
- Exatidão com Uso de Material de Referência
-
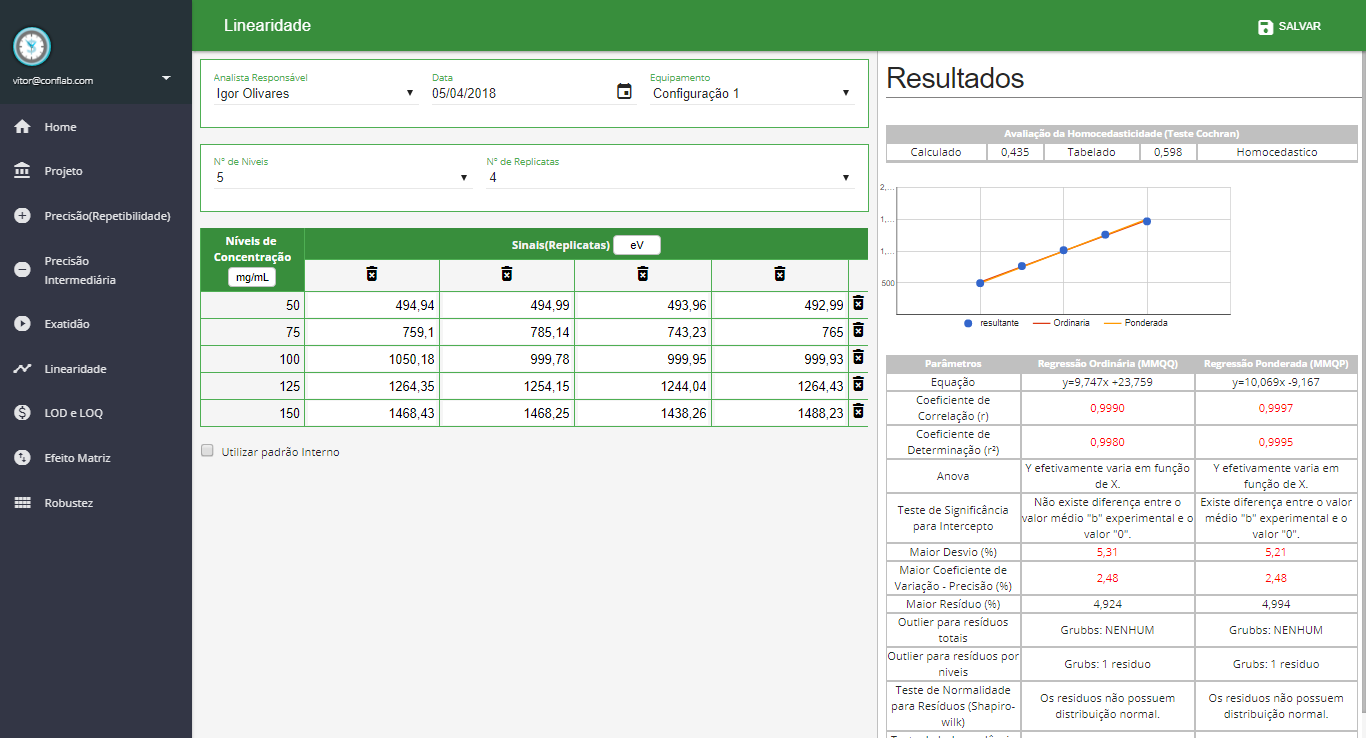
Linearidade
- Avaliação da Homocedasticidade
-
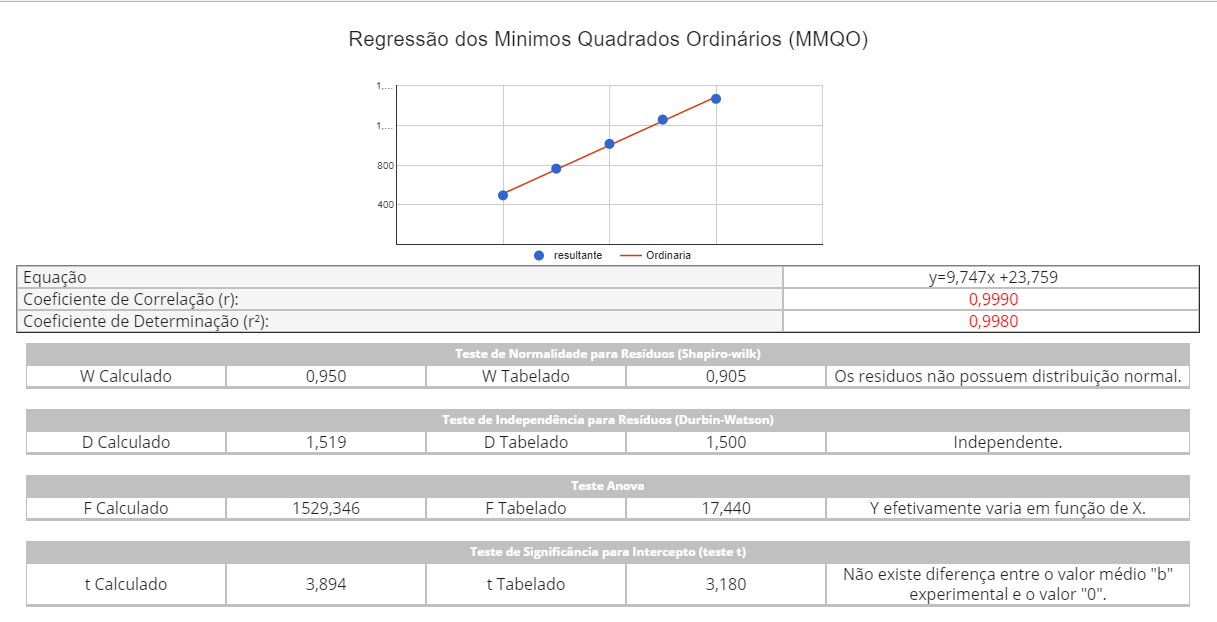
Regressão dos Mínimos Quadrados Ordinários (MMQO) e Regressão dos Mínimos Quadrados Ponderada (MMQP)
- Testes Gerais
- Normalidade
- Independência
- Anova
- Significância do Intercepto
- Resíduos Absolutos
- Resíduos Relativos
- Outliers (Grubbs por Nível)
- Outliers (Grubbs total)
- Exatidão
- Coeficiente de Variação
- Regressão dos Mínimos Quadrados Ordinários (MMQO) Versus Regressão dos Mínimos Quadrados Ponderados (MMQP)
-
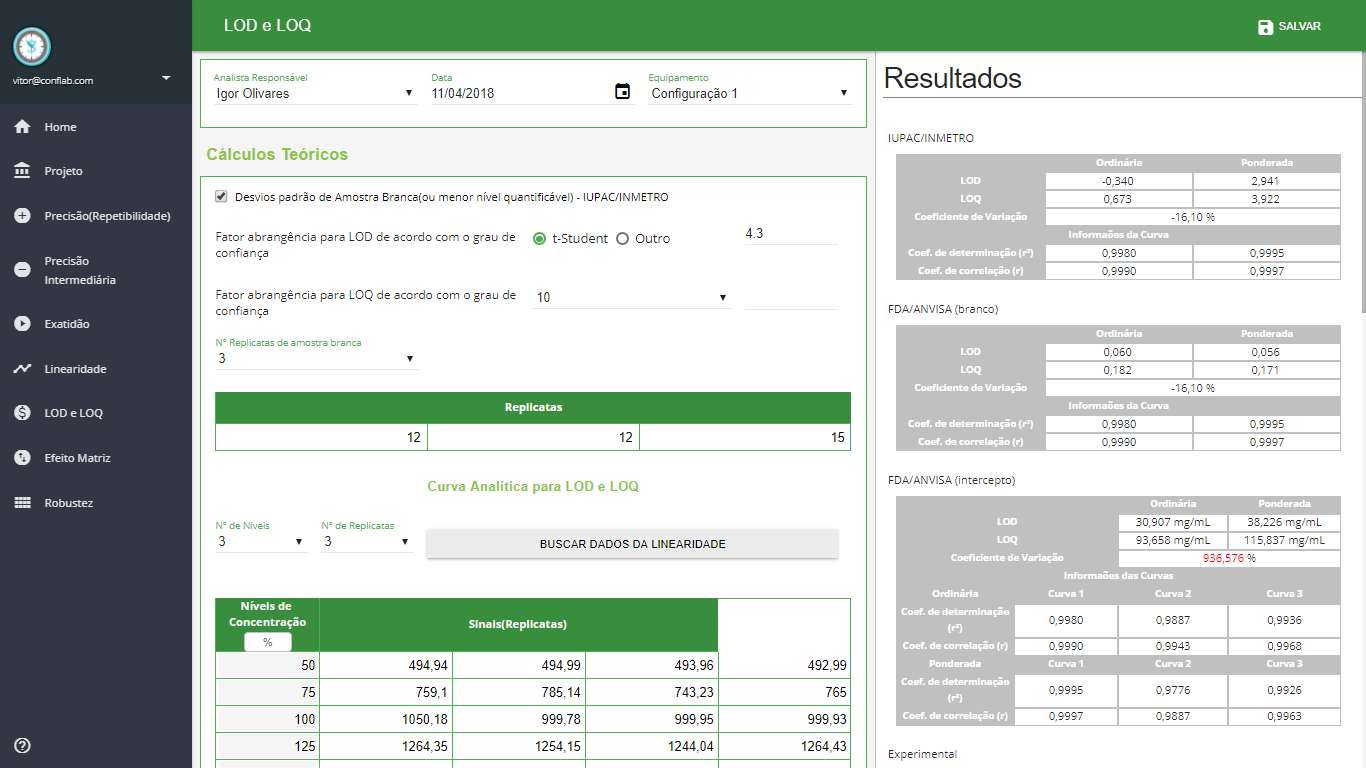
LOD e LOQ
- Cálculos Teóricos - Desvios padrão de Amostra Branca (ou menor nível quantificável) - IUPAC/INMETRO
- Cálculos Teóricos - Desvios padrão de Amostra Branca (ou menor nível quantificável) - FDA/ANVISA
- Cálculos Teóricos - Desvio padrão de Intercepto do Sinal - FDA/ANVISA
- Cálculos Experimentais
-
Efeito Matriz
- Diferenças Entre As Curvas De Calibração
- Desvios Relativos aos FMNs
-
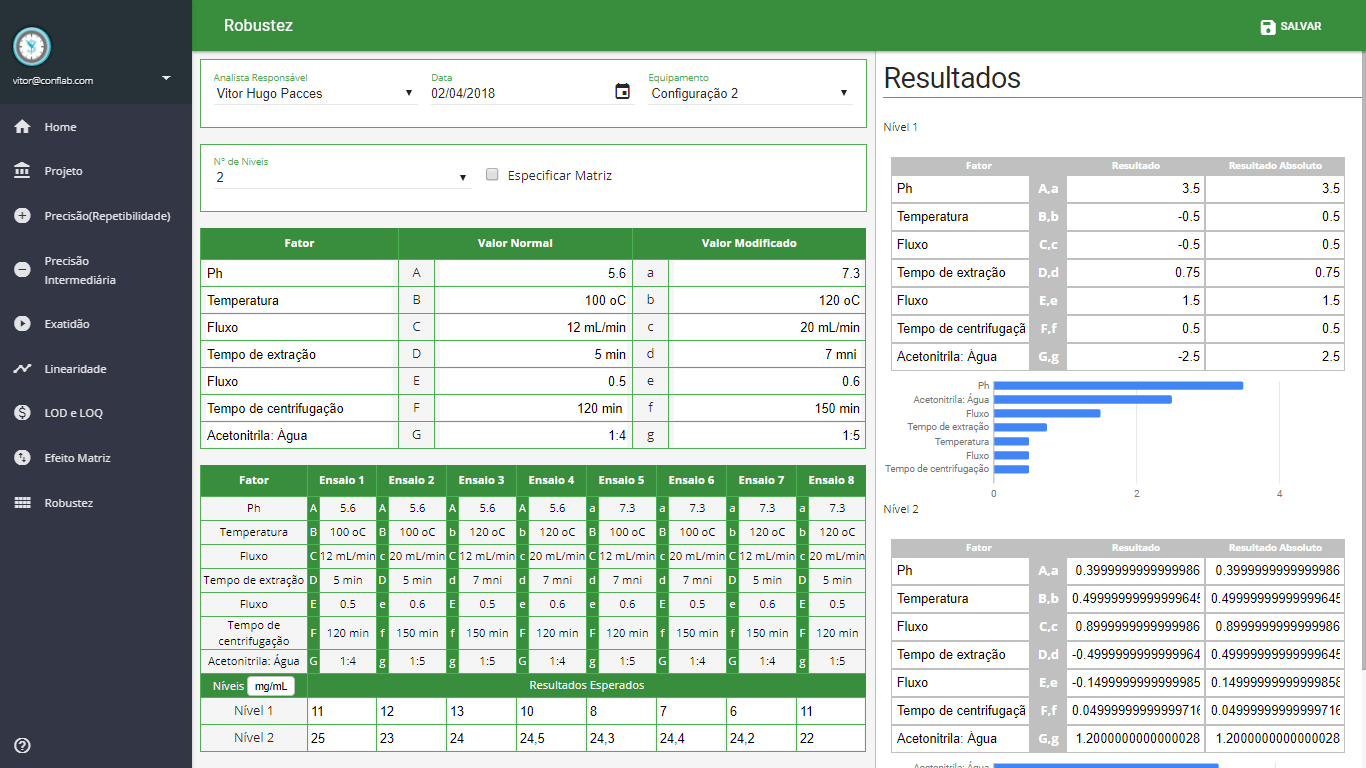
Robustez
- Teste de Youden

Validacao, Incerteza e Controle de Qualidade
Todos os direitos reservados
phone(19) 99176-7122